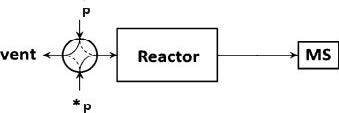
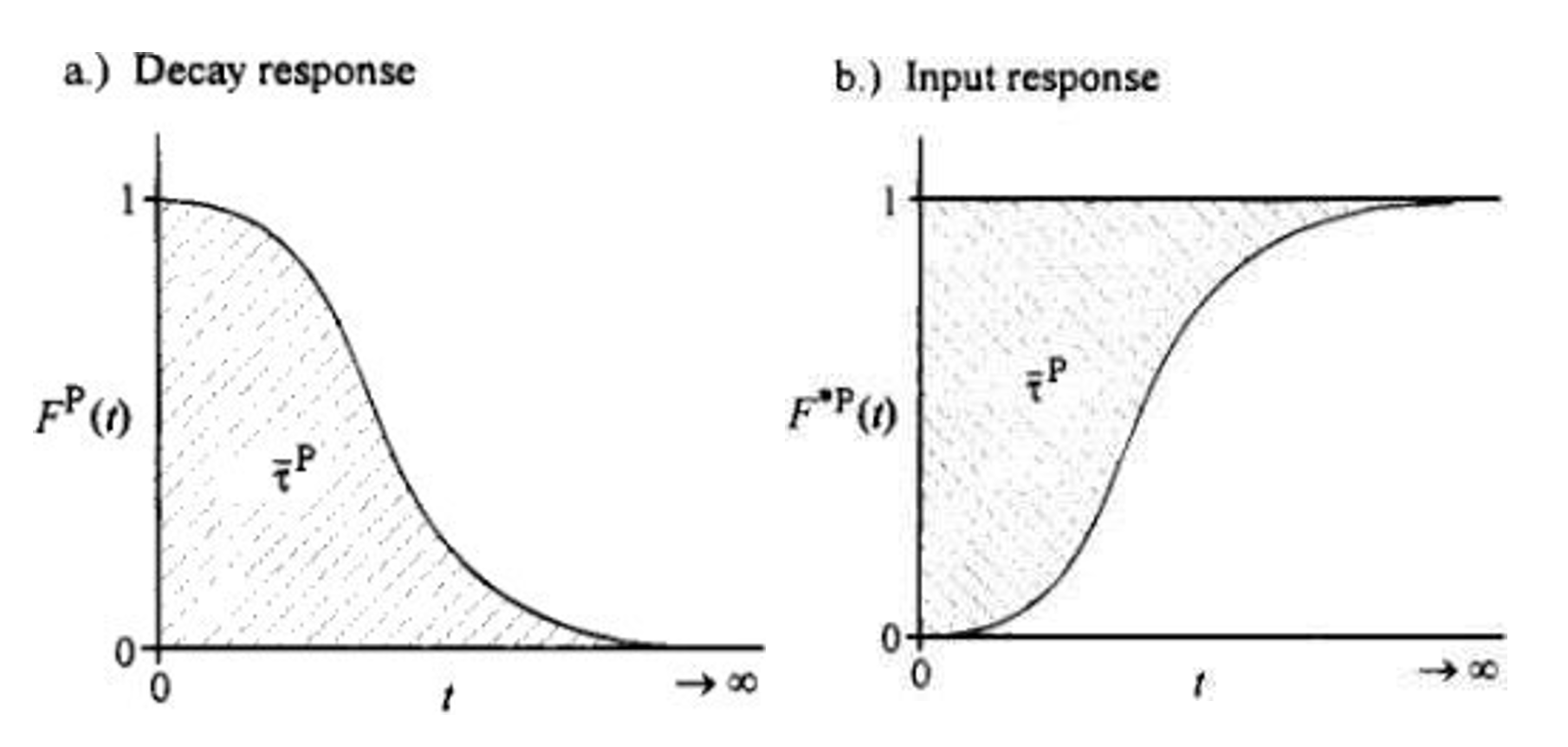
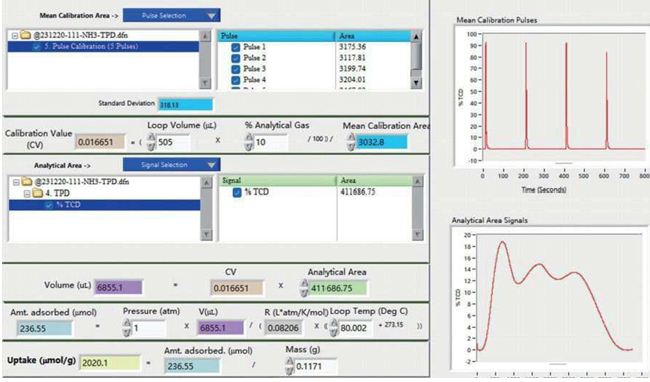
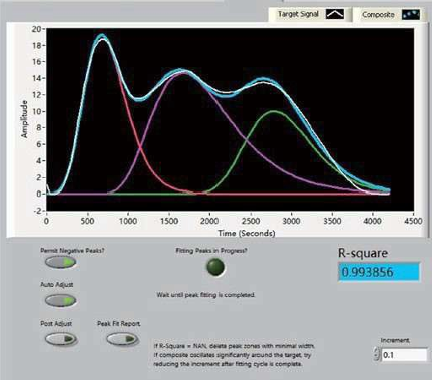
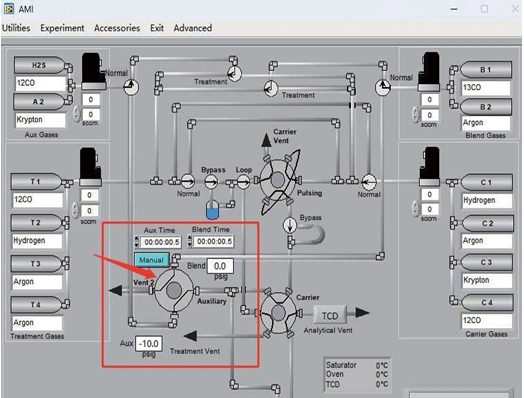
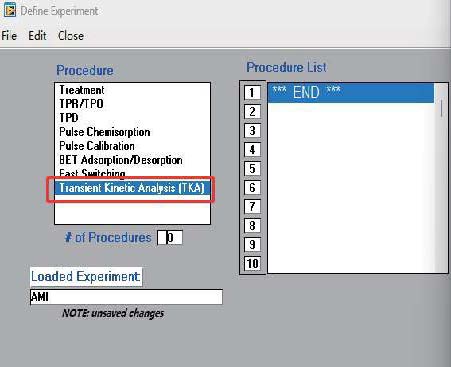
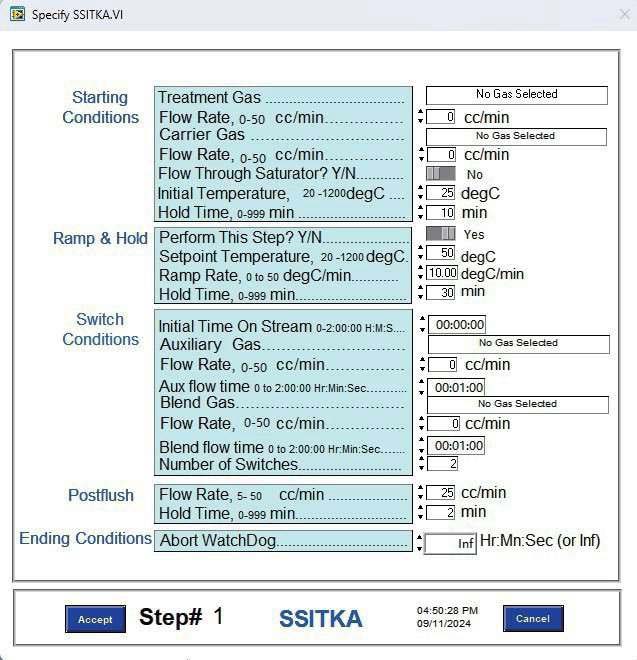
- The AMI-300 SSITKA is a high-performance chemisorption analyzer integrated with Steady-State Isotopic Transient Kinetic Analysis (SSITKA) capabilities. Compared to conventional chemisorption analyzers, the AMI-300 SSITKA employs SSITKA technology to enable in-depth investigation of catalyst reaction mechanisms and properties. The instrument rapidly switches the isotopic composition of a reactant within the reaction system while monitoring the relaxation dynamics of labeled products in real time. This methodology facilitates precise analysis of reaction mechanisms, measurement of kinetic parameters, catalyst characterization, and differentiation of parallel reaction pathways.
Advanced Measurement Instruments
Copyright 2004–2024 ami-instruments.com

 Products
Products
 Products
Products






 TEL: +1 262-877-3600
TEL: +1 262-877-3600
 EMAIL:sales@ami-instruments.com
EMAIL:sales@ami-instruments.com